Abstract
OBJECTIVE:
To establish whether cannabis is an effective and safe treatment option in the management of pain.
DESIGN:
Systematic review of randomised controlled trials.
DATA SOURCES:
Electronic databases Medline, Embase, Oxford Pain Database, and Cochrane Library; references from identified papers; hand searches.
STUDY SELECTION:
Trials of cannabis given by any route of administration (experimental intervention) with any analgesic or placebo (control intervention) in patients with acute, chronic non-malignant, or cancer pain. Outcomes examined were pain intensity scores, pain relief scores, and adverse effects. Validity of trials was assessed independently with the Oxford score.
DATA EXTRACTION:
Independent data extraction; discrepancies resolved by consensus.
DATA SYNTHESIS:
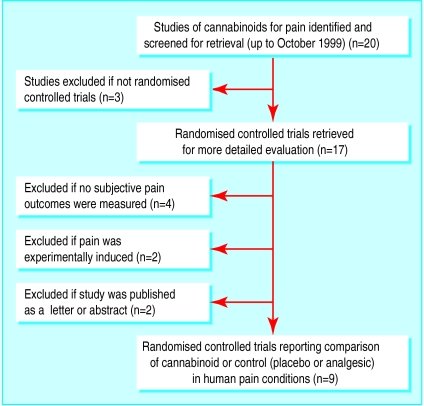
20 randomised controlled trials were identified, 11 of which were excluded. Of the 9 included trials (222 patients), 5 trials related to cancer pain, 2 to chronic non-malignant pain, and 2 to acute postoperative pain. No randomised controlled trials evaluated cannabis; all tested active substances were cannabinoids. Oral delta-9-tetrahydrocannabinol (THC) 5-20 mg, an oral synthetic nitrogen analogue of THC 1 mg, and intramuscular levonantradol 1.5-3 mg were about as effective as codeine 50-120 mg, and oral benzopyranoperidine 2-4 mg was less effective than codeine 60-120 mg and no better than placebo. Adverse effects, most often psychotropic, were common.
CONCLUSION:
Cannabinoids are no more effective than codeine in controlling pain and have depressant effects on the central nervous system that limit their use. Their widespread introduction into clinical practice for pain management is therefore undesirable. In acute postoperative pain they should not be used. Before cannabinoids can be considered for treating spasticity and neuropathic pain, further valid randomised controlled studies are needed.
Source: Pubmed
PMID: 11440935 PMCID: PMC3432
Campbell FA1, Tramèr MR, Carroll D, Reynolds DJ, Moore RA, McQuay HJ.